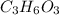Answer:
The molecular formula of the compound =
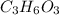
Step-by-step explanation:
Given, The empirical formula is =

Molecular formulas is the actual number of atoms of each element in the compound while empirical formulas is the simplest or reduced ratio of the elements in the compound.
Thus,
Molecular mass = n × Empirical mass
Where, n is any positive number from 1, 2, 3...
Mass from the Empirical formula = 12 + 2×1 + 16 = 30 g/mol
Also, given that:-
89 g/mol < Molar mass > 91 g/mol
So,
Molecular mass = n × Empirical mass
89 g/mol < 30 n > 91 g/mol
⇒ n ≅ 3
The molecular formula of the compound =