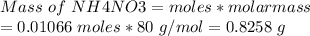Answer:
Mass of solid ammonium nitrate that must be used = 0.853 g
Step-by-step explanation:
Given:
Volume of N2O obtained, V = 0.239 L
Temperature T at STP = 273 K
Pressure P at STP = 1 atm
Calculate the number of moles (n) of N2O obtained based on ideal gas equation:
PV = nRT
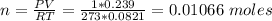
The given reaction is:
NH4NO3(s) -> N2O(g) + 2 H2O(g)
Based on the stoichiometry:
1 mole of NH4NO3 produces 1 mole of N2O
Therefore, from the above calculation:
moles of NH4NO3 needed = 0.01066 moles
Molar mass of NH4NO3 = 80 g/mol