Answer:
The correct answer is : 5 mL of acetic acid/100 mL of solution
Step-by-step explanation:
The 5 percent (v/v) measurement tells us about volume of the pure acetic acid in an overall solution.
Suppose in 1000 mL of solution , the volume of acetic acid be x
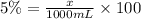
x = 50 mL
Similarly in 100 ml of solution, let the of acetic acid volume be y.
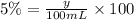
y = 5 mL