Answer: 58 years
Explanation: -
Radioactive decay follows first order kinetics.
Half-life of strontium-9 = 29 years

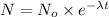
N = amount left after time t = 0.25 kg
 = initial amount = 1 kg
= initial amount = 1 kg
 = rate constant = 0.024
= rate constant = 0.024
t= time = ?


Thus it takes 58 years to a 1 kg sample of strontium-90 decay and reduce to 0.25 kg of strontium-90.