Answer:
Option a. The number of atoms for each element are the same on the product side and the reactant side
Step-by-step explanation:
Chemical equation:
Chemical equation is a symbolic representation of any chemical reaction in the form of symbols of element and chemical formula of the compounds involved.
All the reactants involved are written on the left hand side and all the products formed are written on right hand side. Reactants and products are separated by arrow.
Balanced chemical equation is required for the stoichiometric calculation.
In balanced chemical equation:
- Number of atoms for each element present towards reactant side is equal to the number of atoms for each element towards product side.
- Charges of reactant side is also equal to the charges present towards produc side.
Consider a balanced chemical equation:
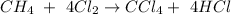
C = 1 C = 1
H = 4 H = 4
Cl = 8 Cl = 8
Among given options, option a is correct.