Answer: The solution contains more hydronium ions than hydroxide ions.
Step-by-step explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydronium ion concentration and pOH is calculated by taking negative logarithm of hydroxide ion concentration.
![pH=-\log [H_3O^+]](https://img.qammunity.org/2017/formulas/chemistry/high-school/m8izixgjxqx2sqg3deswezu589c0fets04.png)


Given,
![[H_3O^+]=1.56* 10^(-4)M](https://img.qammunity.org/2017/formulas/chemistry/high-school/hib58qjkicxog976o66b5b71mlwyuzj08v.png)
![pH=-\log [1.56* 10^(-4)]](https://img.qammunity.org/2017/formulas/chemistry/high-school/7jbk0phnpgc1t0damtykdml0eeomb4p5xm.png)
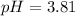

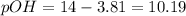
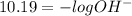
![[{OH^-}]=6.45* 10^(-11)M](https://img.qammunity.org/2017/formulas/chemistry/high-school/s08n4dqxr22elsdilq3r7z5mahjezzqv3z.png)
So
![[H^+]](https://img.qammunity.org/2017/formulas/chemistry/high-school/tus7ihg10qgbkbw5g460sfzwbs8jrjuufg.png) >
>
![[OH^-]](https://img.qammunity.org/2017/formulas/chemistry/high-school/l3faxidumb00bxe58aswem1wyvqt1iv43l.png)