Answer: Option (b) is the correct answer.
Step-by-step explanation:
According to ideal gas equation, the product of pressure and volume is equal to the product of universal gas constant and absolute temperature.
Mathematically, PV = nRT ........... (1)
where P = pressure
V = volume
n = number of moles
R = gas constant
T = temperature
Also, number of moles =

Therefore, number of moles of oxygen atom will be as follows.
Number of moles =

=

=
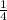
Thus, putting the value of n into equation (1) as follows.
PV = nRT
or PV =
