Answer:
The volume of the larger cube is 5.08 g/cm³.
Step-by-step explanation:
Given that,
Mass of smaller cube = 20 g
Density of smaller cube
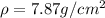
Dylan has two cubes of iron.
The larger cube has twice the mass of the smaller cube.
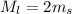
Density is same for both cubes because both cubes are same material.
The density is equal to the mass divided by the volume.

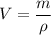
Where, V = volume
m = mass
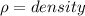
We need to calculate the volume of smaller mass
The volume of smaller mass
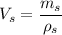
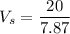

Now, We need to calculate the volume of large cube
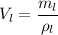
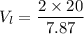
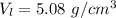
Hence, The volume of the larger cube is 5.08 g/cm³.