To solve this question we need to use the combined gas law:
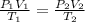
sufix 1 reffears to the initial state and sufix 2 reffears to the final state. P is pressure, V volume and T temperature (in Kelvin). They are asking for the pressure at the final state (P2) and they give the initial conditions: P1= 1atm, V1=1L T1=25°C=298K and the final temperature T2=1000°C= 1273 K. They alse say that the Volume is fixed so V2=V1=1L. then we just have to substitute the values into the eqaution:
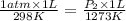
Then solve P2. P2 = 4.272 atm