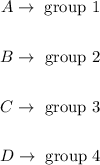Step 1 - Finding the molecular formulas for each compound
We can see each compound is made up of H, C and O atoms. Therefore, its molecular formula will be something like:
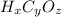
What we need to do to discover the full formula is counting the number of H, C and O atoms in the drawing. Let's do it for each compound:
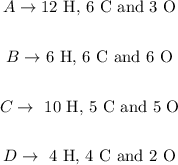
The molecular formulas will be thus:
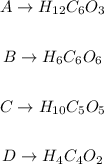
Step 2 - Finding the empirical formula for each compound
The empirical formula can be obtained by dividing the molecular formula by a number that divides all other numbers. We have to do this because in the empirical formula we express only the least possible proportion between the elements.
For example, in compound A, H12C6O3, all numbers (12, 6 and 3) are divisible by 3, therefore the empirical formula for A is:
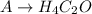
Doing the same for all others:
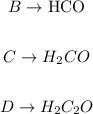
Step 3 - Sorting the compounds in groups
Since we have not obtained any compound with the same empirical formula, each compound belongs to its own group. Therefore we could sort it as: