The given reaction is already balanced, that is to say tha the number of atoms in the reactants matches the number of atoms in the products. In the reaction, we can see the relationship between CaC2 and H2O. For each mole of CaC2 two moles of H2O react.
So, if 4.8 moles of CaC2 are consumed the moles of H2O needed will be:
Mol of H2O = Mol of CaC2 x 2
Mol of H2O = 4.8 x 2 = 9.6 mol of H2O
Now, to calculate the grams of H2O we will use the following equation and the mass molar of H2O.
Mass molar of H2O =18.01 g/mol
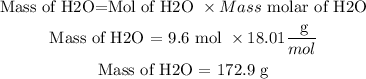
So, if 4.8 moles of CaC2 are consumed in this reaction, 172.9 g of H2O are needed