The given compound is Calcium Nitrate, which is formed by combing Calcium, Oxygen and Nitrogen atoms.
In the compound

,the subscript, 3 implies that, there are three atoms of oxygen in the parenthesis.
The 2 outside the parenthesis tells us the nitrate

is doubled and that each atom is also doubled.
In order to obtain the number of oxygen atoms in the compound, we have to multiply the subscript,
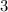
of oxygen, by the subscript
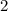
which is outside the parenthesis.
This implies that,
The number of oxygen atoms

Therefore there are

oxygen atoms in the given compound.