So,
Let's begin with the first compound.
We're given the following ions:
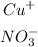
What we need to do to find the empirical formula of the compound formed by these ions, is to change the numbers of the ionic charges by the number atoms of the other compound. Let me explain this with a drawing:
In this case, both charges are 1, so, the ionic compound formed is Cu(NO3). The name of this compound is Copper(I) nitrate.
Let's continue with the second one:
So, the formed compound is:
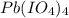
This compound is called Lead(IV) Periodate.
And finally, let's do the third one:
Thus, the empirical formula of this compound will be:
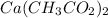
The name of this compound is Calcium Acetate.