The concentration : 0.569 M
Further explanation
Given
Mass of NaCl : 49.9 g
Volume = 1.5 L
Required
The concentration
Solution
mol NaCl :
= mass : MW
= 49.9 : 58.5
= 0.853
Molarity shows the number of moles of solute in every 1 liter of solute or mmol in each ml of solution
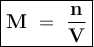
Input the value :
M = 0.853 mol : 1.5 L
M = 0.569