Answer
16.7 g
Step-by-step explanation
Given that:
Volume of KOH = 554 mL = 0.554 L
Molarity of KOH = 0.652 M
Volume of NiSO4 = 518 mL = 0.518 L
Molarity of NiSO4 = 0.580 M
What to find:
The mass of the precipitate formed.
Step-by-step solution:
Step 1: Write the balanced equation for the reaction.
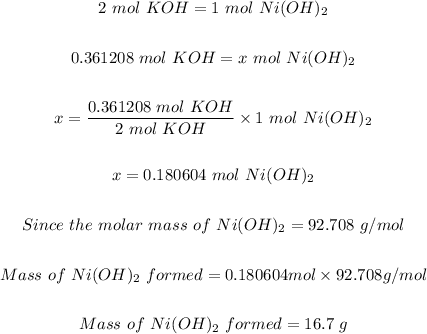
2KOH(aq) + NiSO4(aq) → Ni(OH)2(s) + K2SO4(aq)
The precipitate formed is solid Ni(OH)₂
Step 2: Convert the reactants to moles
0.554 L of 0.652 M KOH to mole is:
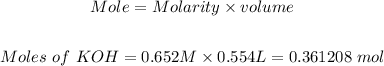
0.518 L of 0.580 M NiSO4 to mole is:
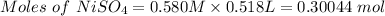
Step 3: Determine the limiting reactant.
From step 1; the mole ratio of KOH to NiSO4 is 2:1
So comparing the mole ration with the moles of KOH to NiSO4 in step 2, KOH would be the limiting reactant.
We can now use the limiting reactant (KOH) to calculate the mass of precipitate formed.
Step 4: Calculate the mass of the precipitate (Ni(OH)₂) formed.