Answer: The volume of solvent evaporated is 173.57 mL
Step-by-step explanation:
 .....(1)
.....(1)
Molarity of solution = 0.275 M
Volume of solution = 230.0 mL
Putting values in equation 1, we get:
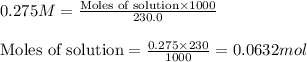
As, the moles of solution remains the same.
When solvent gets evaporated, the volume of the solution is calculated by using equation 1:
Moles of solution = 0.0632 moles
Molarity of the solution = 1.15 M
Putting values in equation 1, we get:
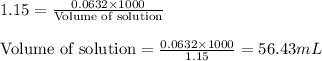
Volume of solution evaporated = (230.0 - 56.43) mL = 173.57 mL
Hence, the volume of solvent evaporated is 173.57 mL