Answer:
The molarity of the solution is 1.23M.
Step-by-step explanation:
1st) To calculate the molarity, it is necessary to have the moles of the solute per liter (or 1000mL) of solution. So, we have to convert 1.20g of HCl to moles, using the molar mass of HCl (36.5g/mol):
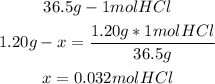
2nd) Now we know that 0.032 moles of HCl are contained in 25.0mL of water.
HCl is the solute and water is the solvent, so with the density of HCl (1.3g/mL) we have to calculate the mL of HCl:
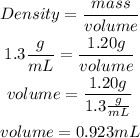
Now, adding the solute plus the solvent, we get the volume of the solution:
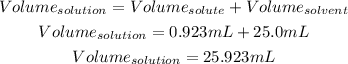
3rd) Finally, we can calculate the molarity of the solution with a mathematical rule of three:

So, the molarity of the solution is 1.23M.