Step-by-step explanation:
Combustion is defined as the reaction in which a hydrocarbon reacts with oxygen and leads to the formation of carbon dioxide and water.
For example,
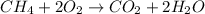
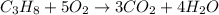
According to law of conservation of mass, mass of reactants will be equal to the mass of products formed because mass can neither be created nor it can be destroyed. It simply changes into one form to another.
Thus, we can conclude that when a hydrocarbon undergoes combustion, it reacts with oxygen and produces carbon dioxide and water.