The final volume : 807.69 cm³
Further explanation
Given
Initial volume : 840 cm³
Initial pressure : 750 mmHg
Final pressure : 780 mmHg
Required
The final volume
Solution
Boyle's Law
At a fixed temperature, the gas volume is inversely proportional to the pressure applied
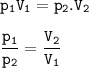
Input the value :
V₂=(P₁V₁)/P₂
V₂=(750 x 840)/780
V₂=807.69 cm³