Answer:
36°C
Step-by-step explanation:
Given parameters:
Mass of aluminum = 725g
Quantity of heat = 2.35 x 10⁴J
Unknown:
Temperature change = ?
Solution:
To solve this problem, we simply use the expression below:
The quantity of energy is given as:
Q = m C Δt
Q is the quantity of energy
m is the mass
C is the specific heat capacity of aluminum = 0.9J/g°C
Δt is the change in temperature
The unknown is Δt;
Δt =
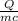 =
=
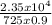 = 36°C
= 36°C