The maximum temperature : a. 745 K
Further explanation
Given
Gas at 25°C(298 K) and at a pressure of 0.800 atm
Final pressure = 2 atm
Required
Final temperature
Solution
Gay Lussac's Law
When the volume is not changed, the gas pressure in the vessel is proportional to its absolute temperature
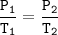
Input the value :
T₂=(P₂T₁)/P₁
T₂=(2 atm x 298 K)/0.8 atm
T₂ = 745 K = 472 °C