Answer:
C₂H₂O₄
Step-by-step explanation:
Given parameters:
Mass of organic acid = 5g
Mass of C = 1.334g
Mass of O = 3.554g
Mass of H = 5 - (1.334 + 3.554) = 0.112g
Molar mass = 90.03g/mol
Unknown:
Molecular formula of the compound = ?
Solution:
To solve this problem, we find the empirical formula of the compound first. It is the simplest formula of the compound.
Elements C H O
Mass 1.334 0.112 3.554
Molar mass 12 1 16
Number of moles 1.334/12 0.112/1 3.554/16
0.112 0.112 0.222
Divide by the
Smallest 0.112/0.112 0.112/0.112 0.222/0.112
1 1 2
Empirical formula = CHO₂
The molar mass of this empirical formula = 12 + 1 + 2(16) = 45g/mol
Now;
Multiplication index =
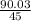 = 2
= 2
Molecular formula = (CHO₂)₂ = C₂H₂O₄