Answer:
When the mass increases or when distance between the bodies reduces
Step-by-step explanation:
According to Newton's law of universal gravitation, the gravitational attraction between two bodies always increase if the mass increases and the distance between the bodies reduces.
The law of universal gravitation states that "the gravitational force of attraction between two bodies is directly proportional to the product of their masses and inversely proportional to the square of the distances between them".
Mathematically;
Fg =
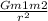
G is the universal gravitation constant
m is the mass
r is the distance