To make the conversion that they ask us, we must take into account that a mole of any substance has 6.022x10^23 molecules, that is Avogadro's number. So the molecules contained in 1.75mol of H2O will be:
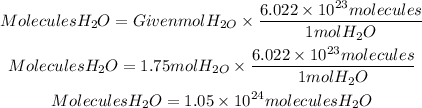
In 1.75 mol of H2O there are 1.05x10^24 molecules of H2O