So, solving for row three, we are given that:
The mass of NaHCO3 is 1.5g.
To convert this amount to moles, we divide by the molecular mass of NaHCO3, which is 84g/mol.
So,
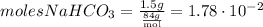
And, given that the moles of C2H3O2H are:
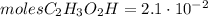
The amount of moles of NaHCO3 is less than the amount of moles of C2H3O2H, so, the limiting reagent is NaHCO3 and the excess reagent is C2H3O2H.