Answer:The initial temperature of the water 62.13 °C.
Step-by-step explanation:
Heat absorbed by the gold :Q
Mass of the gold ,m= 15.23 g
Specific heat capacity of gold = c = 0.129 J/g °C
Change in temperature of the gold =
 =62°C-54°C=8°C
=62°C-54°C=8°C
Heat lost by the water : Q'
Mass of the water,m' = 28 g
Specif heat of water = c' = 4.179 J/g °C
Change in temperature of the water =
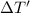
let the initial temperature of the water be T.
As per as Law of Conservation of Energy:
Q = -Q'
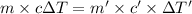
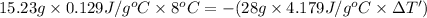
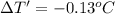
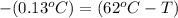
T = 62.13 °C
The initial temperature of the water 62.13 °C.