Answer: The correct answer is Option 1.
Step-by-step explanation:
For the given options:
- Option 1: 3.55 mole of O atoms
To calculate the number of moles, we use the equation:
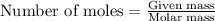
Moles of Oxygen = 3.55 mol
Molar mass of Oxygen = 16 g/mol
Putting values in above equation, we get:
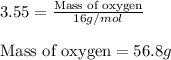
Mass of oxygen atoms = 56.8 g
- Option 2: 4.52 g of O atoms
Mass of oxygen atoms = 4.52 g
- Option 3: 0.0032 kg of
 molecule
molecule
Converting this into grams, we use the conversion factor:
1 kg = 1000 g
So, 0.0032 kg = 3.2 g
Mass of
 molecule = 3.2 g
molecule = 3.2 g
- Option 4:
 of
of
 molecule
molecule
According to mole concept:
1 mole of an atom contains
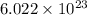 number of atoms.
number of atoms.
If,
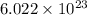 number of atoms occupies 32 grams of oxygen molecule.
number of atoms occupies 32 grams of oxygen molecule.
So,
 number of atoms will occupy =
number of atoms will occupy =
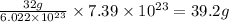
Mass of
 molecule = 39.2 g
molecule = 39.2 g
Hence, the greatest mass is coming out from option 1.