Suppose that there is 'x' oz of solution initially.
Given that the concentration initially was 12%, it means that 12 % of the solution was pure super grow, so the initial mass of pure super grow in the solution is,
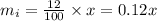
Given that the weight of the final mixture is 32 oz.
And the concentration is given as 17.5%, it means that 17.5% of the solution is pure super grow, so the final mass of pure super grow in the solution is,
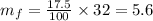
Now, consider that the remaining content of the solution is the solvent only.
Since initially the solution has 0.12x oz os pure super grow, so the initial mass of solvent in the solution should be 0.88x oz.
Similarly, the final solution has 5.6 oz of pure super grow, so the final mass of solvent must be 26.4 oz.
Note that