Answer:The false statements are:
1. Gallium is the catalyst.
2. Gallium is gaining electrons.
Step-by-step explanation:
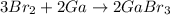
In the above reaction bromine is getting reduced as its oxidation states changes from 0 to -1.
Gallium is losing electrons to bromine due to which its oxidation state changes from 0 to +3. Also catalyst doesn't show up in the product means they only effect rate of the reaction.
Hence ,gallium is not gaining electrons and also not a catalyst.