Answer
D. 2Na + Cl₂ → 2NaCl and 0.2175 moles or 7.71 g
Step-by-step explanation
Given:
Mass of sodium that reacts = 10 g
What to find:
To write the balanced equation then find the number of moles in the mass of chlorine Cl₂ required to react with 10 g of sodium.
Step-by-step solution:
Step 1: Write the balanced chemical equation between the sodium metal and chlorines.
2Na + Cl₂ → 2NaCl
Step 2: Convert the mass of sodium metal given to moles using the mole formula.
Molar mass of Na = 23.0 g/mol
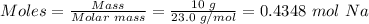
Step 3: Determine the moles of Cl₂ using the moles of Na in step 2 and mole ratio from the equation in step 1.
From step 1; 2 mole of Na requires 1 mole of Cl₂
So, 0.4348 moles of Na will require
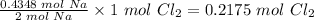
Step 4: Convert the moles of Cl₂ in step 3 to mass using the mole formula.
Molar mass of Cl = 34.453 g/mol
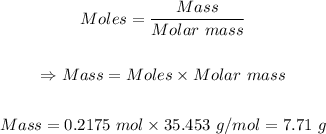
Therefore, the correct answer is:
D. 2Na + Cl₂ → 2NaCl and 0.2175 moles or 7.71 g