Answer:
32 moles of water
Step-by-step explanation:
From the reaction, we can deduce that 1 mole of oxygen gas (O2) was used to produce 2 moles of water (H2O).
Hence, 16 moles of oxygen will produce "X" moles of water (where X is the unknown).
1 mole of O2 ⇒ 2 moles of H2O
16 moles of O2 ⇒ X
X =
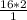 = 32 moles of H2O
= 32 moles of H2O
16 moles of O2 will produce 32 moles of H2O