Answer: The correct option is D.
Step-by-step explanation: To calculate the larges number of partivles of gas, we will use Avogadro's Law which says that volume of the gas is directly related to the number of moles of gas at constant pressure and temperature.
Mathematically,
 ....(1)
....(1)
The gas which has the largest number of moles, will have the largest number of particles.
We are provided to use STP conditions, which says that
1 mole of a gas occupies 22.4 L of volume.
Initial conditions:
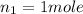
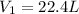
- A. 0.520 L of

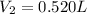
Putting all the values, in equation 1, we get
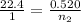
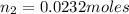
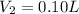
Putting all the values, in equation 1, we get

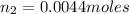
- C. 7.0 L of


Putting all the values, in equation 1, we get
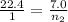
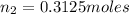
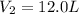
Putting all the values, in equation 1, we get
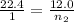
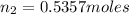
Hence, from the above calculations, we see that option D has the largest amount of moles and hence, will have the largest amount of particles.