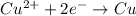Answer: (the reactions are highlighted in the explanation)
Step-by-step explanation:
The question requires us to provide the half-reactions for the following oxidation-reduction reaction:
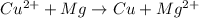
Half-reactions in a redox reaction corresponds to the separated reduction and oxidation reaction. Thus, we need to identify the elements that are oxidizing and reducing and how many electrons are involved in these process in order to find the half-reactions.
Keep in mind that in an oxidation process, the element loses electrons and its oxidation number increases, while in a reduction process, the element gains electron and its oxidation number reduces.
In the complete redox reaction given, note that the oxidation number of Cu changes from +2 (in the reactants) to 0 (in the products), while the oxidation number of Mg changes from 0 to +2. Therefore, Cu is being reduced while Mg is being oxidized, and, since the oxidation numbers changed by 2, there are 2 electrons involved in both oxidation and reduction processes.
Therefore, we can write the oxidation half-reaction as:
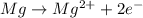
And the reduction half-reaction can be written as: