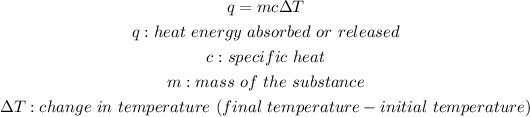Answer: Calorimetry simply put is the measurement of heat. A calorimeter works by capturing all the energy that is absorbed or released. The energy that leaves one system is equivalent to the energy that is absorbed by the system provided that energy is not loss. The heat given off is calculated similarly to the heat absorbed what makes the difference is that heat given of is negative whilst heat gain is positive. The formula for heat absorbed or released can be written as: