Answer:
The correct answer is option A.
Step-by-step explanation:
Initial volume of the gas

Initial temperature of gas

Final volume of the gas

Final temperature of the gas =

Applying Charles' Law:
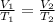

The temperature of the gas when volume of the gas is 20.0 L is 160 K.Hence, the correct answer is option A.