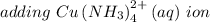Answer
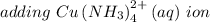
Explanation
The given equation is
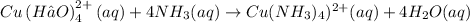
From the information provided, the species/reactant responsible for the pale sky-blue color on the left side of the reaction is the coordination compound
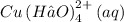
And the species/reactant responsible for the dark blue color on the right side of the reaction is the coordination compound
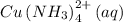
Therefore a change to a solution at equilibrium that will make the solution darker is