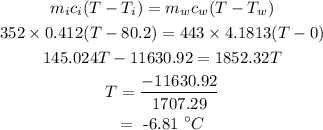Given:
The mass of the iron is
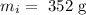
The specific heat of iron is
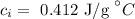
The initial temperature of the iron is
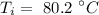
The mass of the water is
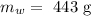
The specific heat of water is
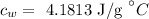
The initial temperature of the water is
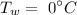
Required: Final equilibrium temperature.
Step-by-step explanation:
Let the final equilibrium temperature be T.
The final equilibrium temperature can be calculated as