Answer: C) Isotopes differ only in their number of neutrons - so they contain 0, 1, and 2 neutrons, respectively.
Explanation: Isotopes are elements which have same atomic number but different mass number. Hydrogen has three isotopes named as hydrogen
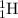 , deuterium
, deuterium
 , and tritium
, and tritium
 .
.
Atomic number= number of protons = number of electrons (for neutral atom)
Mass number = number of protons + number of neutrons
Thus Number of neutrons = Mass Number - Atomic number
Thus number of neutrons in hydrogen with atomic number 1 and mass number 1 = 1 - 1 = 0
Thus number of neutrons in deuterium with atomic number 1 and mass number 2 = 2 - 1 = 1
Thus number of neutrons in tritium with atomic number 1 and mass number 3 = 3 - 1 = 2