Answer:
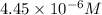 is the concentration of the solution in molarity.
is the concentration of the solution in molarity.
The concentration of fluorescein in solution is 1.88 ppm.
Step-by-step explanation:

Moles of fluorescein =
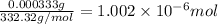
Volume of the fluorescien ethanol solution = 225 mL = 0.225 L
1 mL = 0.001 L
Molarity of the solution :
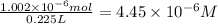
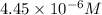 is the concentration of the solution in molarity.
is the concentration of the solution in molarity.

Both the masses are in grams.
Mass of solute that is flouroscein = 0.000333 g
Mass of ethanol = m
Density of the ethanol = d = 0.785 g/mL
Volume of the ethanol = 225 mL
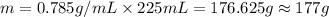
Mass of solution = 0.000333 g + 177 g = 177.000333 g
Concentration in ppm:

The concentration of fluorescein in solution is 1.88 ppm.