Answer:
 photons are produced in a laser pulse of 0.497 Joules at 469 nm.
photons are produced in a laser pulse of 0.497 Joules at 469 nm.
Step-by-step explanation:
Energy of the laser pulse ,E'= 0.497 J
Number of photons = n
Energy of 1 photon with 469 nm wavelength = E
E' = n × E

h = Planck's constant =
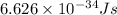
c = speed of light =
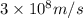
 = wavelength =
= wavelength =


Now put all the given values in the above formula, we get the energy of the photons.



 photons are produced in a laser pulse of 0.497 Joules at 469 nm.
photons are produced in a laser pulse of 0.497 Joules at 469 nm.