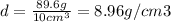Answer:
Density of Copper = 8.96 g/cm3
Step-by-step explanation:
Given:
Mass of copper, m = 89.6 g
Volume of copper, V = 10 cm³
To determine:
The density d of copper
Step-by-step explanation:
The density of a substance is the mass occupied per unit volume. It is expressed as follows:
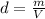
In the case copper: