Answer
132.9 grams
Step-by-step explanation
Given:
Specific heat of the gold metal, c = 0.222 cal/g°C
Quantity of heat energy, Q = 587.0 cal
Initial temperature, T₁ = 18.9 °C
Final temperature, T₂ = 38.8 °C
Temperature change, ΔT = T₂ - T₁ = 38.8 °C - 18.9 °C = 19.9 °C
What to find:
The mass (in grams) of the piece of gold.
Step-by-step solution:
The mass (in grams) of the piece of gold can be calculated using the formula below;
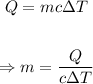
Putting the values of the parameters into the formula, we have
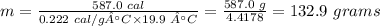
The mass (in grams) of the piece of gold is 132.9 grams.