Answer : The mole ratio of
 to
to
 is, 4 : 2
is, 4 : 2
Explanation :
Mole ratio : It is defined as the ratio of number of moles of the substances whose ratio is to be calculated.
The given balanced chemical reaction is :
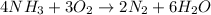
By stoichiometry of the reaction we can say that,
4 moles of ammonia reacts with the 3 moles of oxygen to produce 2 moles of nitrogen and 6 moles of water.
Hence, the mole ratio of
 to
to
 is, 4 : 2
is, 4 : 2