Answer:
E. 19.97 days.
Explanation:
The function that models this situation is
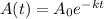
This expression models the decay behaviours, where
 is the decay constant.
is the decay constant.
Now, 5% of its original value refers to
 .
.
Using all this information in the formula, we have
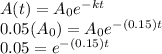
Here, we need to use logarithms to eliminate the power
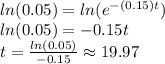
Therefore, the right answer is E. 19.97 days.