Answer :
(1) Mass of Al lost = 0.31 g
(2) The moles of Al lost = 0.0114 mole
(3) Mass of Pb = 3.34 g
(4) The moles of Pb formed = 0.016 mole
Explanation :
Solution for part (1) :
Mass of Al lost = Mass of Al wire before reaction - Mass of Al wire after reaction
Mass of Al lost = 3.96 g - 3.65 g = 0.31 g
Solution for part (2) :

The moles of Al lost = 0.0114 mole
Solution for part (3) :
As we know that,
Mass of Pb + Filter paper = 4.26 g
Mass of Pb = 4.26 g - Mass of filter paper
Mass of Pb = 4.26 g - 0.92 g
Mass of Pb = 3.34 g
Solution for part (4) :
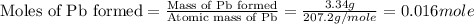
The moles of Pb formed = 0.016 mole