Answer:
1.94 atm is the pressure at underwater site.
19.4 meters is the depth of the underwater site.
Step-by-step explanation:
Pressure of the balloon at the dept at which scuba diver is working:
=
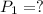
Volume of the balloon at the dept at which scuba diver is working:
=
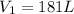
Pressure of the balloon at the surface:
=
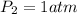
Volume of the balloon at the dept at which scuba diver is working:
=
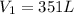
Using Boyle's law:
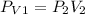 (at constant temperature)
(at constant temperature)
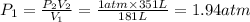
1.94 atm is the pressure at underwater site.
Pressure increases by exactly 1.0 atm for every 10 m depth.
Pressure increase for every 1 unit increase in depth=
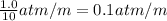
Depth at which pressure is 1.94 atm = h

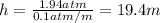
19.4 meters is the depth of the underwater site.