Rydberg equation is
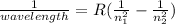
R is the Rydberg constant which is equal to 1.096776 x 10^7 m^-1. Wavelength is 1.094 x 10^-6 m.
So the equation becomes,
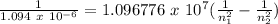
This is trial and error. Our basis should be: n2>n1 and both are whole numbers. According to quantum theory, electrons jump in discrete energies, so there is no in-between. Energy levels must be in a specific quantum number. Then, in order to absorb light, the photon must be able to go to a higher energy level.
If n1 = 1, n2 = 1.04
n1 = 2, n2 = 2.45
n1 = 3, n2 = 6
Hence, n1 = 3 and n2 = 6.