Answer:
137.18mL
Explanations:
To get the required volume, we will use the dilution formula expressed as:
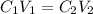
C1 and C2 are the concentration of the solution
V1 and V2 are the corresponding volumes
Given the following parameters:
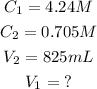
Substitute the given parameters into the formula:
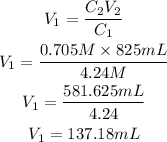
Hence the required volume of KCL that must be diluted to make 825 milliliters of a 0.705 M KCl solution is 137.18mL