Answer: This reaction is a type of synthesis reaction.
Step-by-step explanation: When iron reacts with oxygen which is available in air, it leads to the formation of a reddish-brown compound known as rust.
This reaction is considered as oxidation-reduction reaction because iron is getting oxidized to
 and oxygen is getting reduced to oxide ion.
and oxygen is getting reduced to oxide ion.
Equation of the rust follows:
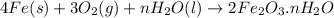
Synthesis reactions are defined as the reactions in which two or more than two reactants combine together to form a single product which can be a molecule or a compound.
The rusting of iron is also considered as a synthesis reaction because iron and oxygen are combining together to form a single compound known as rust.
Both these reactions are a type of Chemical reactions.