Answer:The correct answer si option B.
Step-by-step explanation:
According to Arrhenius equation:
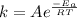
k = Rate constant of the reaction at temperature T.
A = frequency factor
 = Activation energy.
= Activation energy.
R = universal gas constant
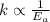
The graph clearly tells us that the activation energy of the reaction A is more than the reaction B.
This means that rate constant of the reaction A is smaller than the rate constant of the reaction B according Arrhenius equation.
As we know that higher the value of rate constant more will be the rate of the reaction.Then the rate of reaction B is more than that of the rate of reaction A.
Hence, the the correct answer is option B.